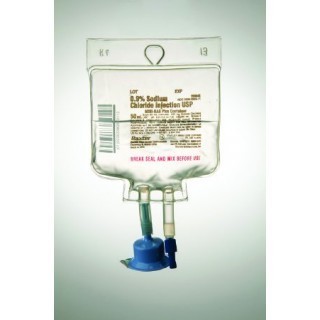
The Baxter 2B0042 is a 0.9% Sodium Chloride Injection, USP, housed in a 50 mL MINI-BAG Plus Container. It is a sterile, nonpyrogenic solution intended for intravenous administration after admixture with a single dose powdered or liquid drug vial. The product is designed to serve as a source of water and electrolytes and is also used as a diluent for reconstitution of a powdered or liquid drug product.
The MINI-BAG Plus Container is a standard diluent container with an integral drug vial adaptor, allowing for drug admixture after connection to a single dose powdered or liquid drug vial with a 20 mm closure. The container is designed to prevent the transfer of contaminants into and out of the system during and after docking, minimizing environmental and personal exposure.
The VIAFLEX Plastic Container is made from a specially formulated polyvinyl chloride (PL 146 Plastic) which is biologically inert and non-DEHP. It can resist heat up to 25 degrees Celsius during a period of 30 days until expiration once opened.
The product is indicated for use as a source of water and electrolytes and may also be used as a diluent for reconstitution of a powdered or liquid drug product packaged in a vial with a 20 mm closure. It is capable of inducing diuresis depending on the clinical condition of the patient.
Please note that the product should be stored at 25 degrees Celsius for up to 1 month without any plastic overwrapping, or until the expiration date. It is sold in a case of 50 units.